Providing and administering RLT
This section of the Novartis RLT Institute is designed to help health care professionals (HCPs) understand radiation safety procedures for providing and administering radioligand therapies (RLTs) manufactured by Novartis. It is based on guidelines from regulatory agencies, including the Nuclear Regulatory Commission (NRC), the Department of Transportation (DOT), and the United States Pharmacopeia (USP). Topics include safe transport, administration, and postadministration protocols to prepare the patient and room for subsequent use.
Overview
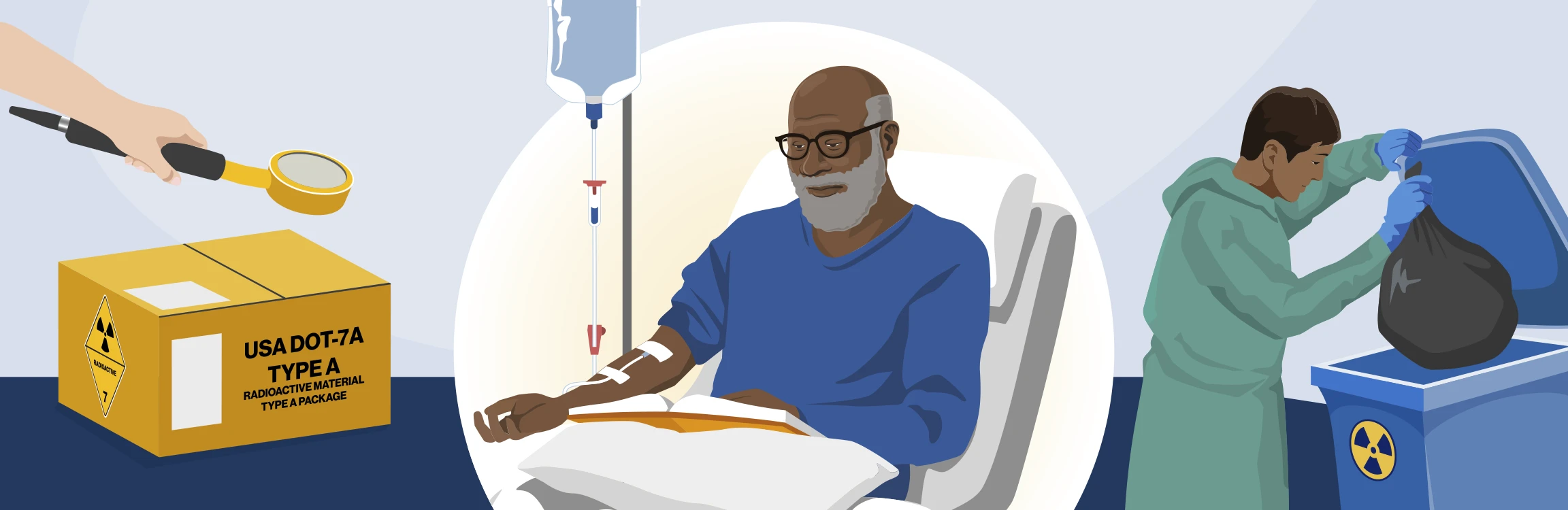
From receipt to patient release: The journey of a dose

Check-in
Upon receiving the RLT pharmaceutical package, it is inspected for integrity and tested for contamination. After passing these checks, the RLT quantity is verified and recorded in the dose inventory system1

Dose preparation
The dosage is prepared for administration as needed, following Food and Drug Administration (FDA) labeling and USP standards for the approved administration method, then transported to the administration area2-5

Infusion
The prepared dosage is administered to the patient in accordance with FDA labeling and USP standards for the approved administration method3,4,6,7

Discharge and decay
After treatment, the patient is discharged with radiation safety instructions and radioactive material (RAM) waste is managed3,8
The information herein is intended to assist practitioners in providing appropriate care for patients when using radiopharmaceuticals. This information is not intended to establish, nor should it be used to establish, a legal standard of care. It is important to adhere to all applicable standards when handling and administering radiopharmaceuticals. Institutions are responsible for ensuring their compliance with all laws and regulations by establishing their own policies, procedures, and guidance. Novartis Pharmaceuticals Corporation does not endorse or recommend any specific course of action, or any third-party organization referenced herein.
