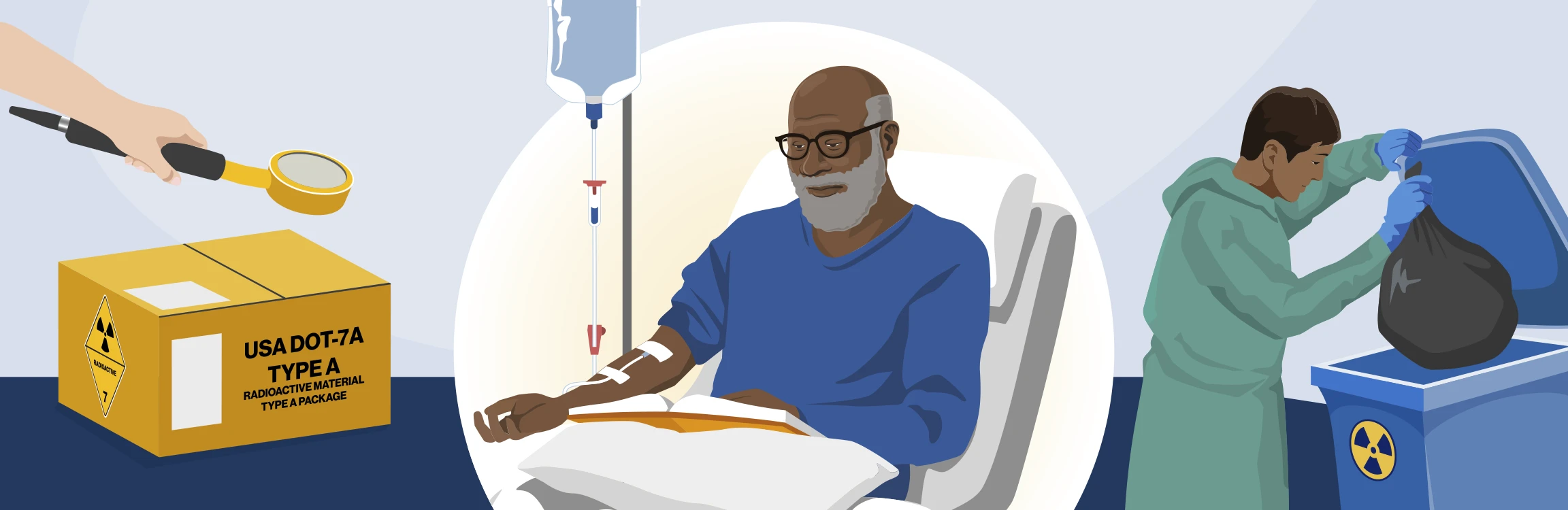
Providing and administering RLT
This section of the Novartis RLT Institute is designed to help health care professionals (HCPs) understand radiation safety procedures for providing and administering radioligand therapies (RLTs) manufactured by Novartis. It is based on guidelines from regulatory agencies, including the Nuclear Regulatory Commission (NRC), the Department of Transportation (DOT), and the United States Pharmacopeia (USP). Topics include safe transport, administration, and postadministration protocols to prepare the patient and room for subsequent use.
Receiving, handling, and storage
Receiving

1
RLTs are transported in Type A packages. Type A packages are designed for shipping radioactive materials (RAM) with higher concentrations than allowed in industrial packaging, include an inner containment vessel surrounded by packing materials, and must meet testing requirements to ensure they retain containment integrity and shielding in transport.1,2 RLTs are typically supplied by the manufacturer in an appropriate radiation shielding container, which is then placed inside a sealed plastic container for shipping.3,4

2
The shipment package and radiation shielding container are evaluated at a distance of 1 m from all sides to ensure there is no radiation contamination.5,6
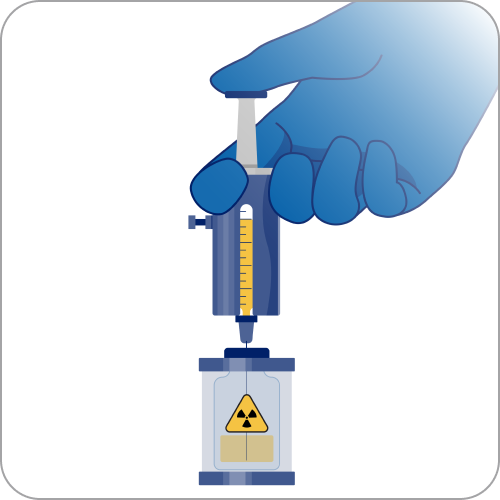
3
If the RLT is delivered in a vial, an authorized individual, such as an authorized nuclear pharmacist or an authorized user (AU), or a nuclear medicine technologist working under the supervision of an AU, may prepare and dispense the dosage.7,8 The site may also receive a prefilled syringe instead.8

4
The amount of radioactivity is confirmed, and the dosage is logged into the site’s inventory system. The remaining amount of radioactivity is measured and logged again after administration, which supports the record-keeping needing to adhere to possession limits stipulated in the RAM license.5,9
